

- Home
- Companies
- Comsol Inc.
- Software
- Comsol - Battery Design Module Software
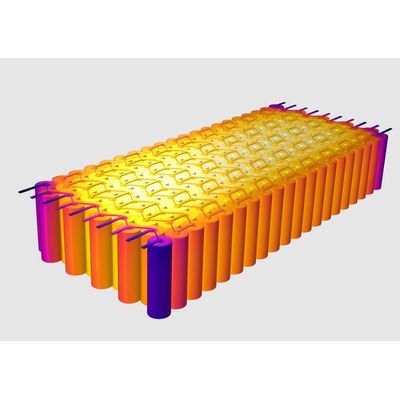
Comsol - Battery Design Module Software
Modeling batteries requires different levels of detail depending on the purpose of the simulations. The Battery Design Module is an add-on to the COMSOL Multiphysics® software that encompasses descriptions over a large range of scales, from the detailed structures in the battery`s porous electrode to the battery pack scale including thermal management systems. The descriptions involve physics phenomena such as transport of charged and neutral species, charge balances, chemical and electrochemical reactions, Joule heating and thermal effects due to electrochemical reactions, heat transfer, fluid flow, and other physical phenomena that are important for the understanding of a battery system.
Lithium-Ion Batteries
The Battery Design Module features state-of-the-art models for lithium-ion batteries. You will find different mechanisms for aging and high-fidelity models, such as the Newman model, available in 1D, 2D, and full 3D. In addition to modeling electrochemical reactions on their own, you can combine them with heat transfer and account for the structural stresses and strains caused by the expansion and contraction from lithium intercalation. The module also provides functionality for setting up heterogenous models, which describe the actual shapes of the pore electrolyte and electrode particles. Studying the microstructure of a battery helps to provide a deeper understanding of the battery performance.
Lead–Acid Batteries
For the simulation of lead–acid batteries, the software includes the dependent variables for ionic potential and composition of an electrolyte and the electric potential and porosity in the solid electrodes. The model accounts for the dissolution and deposition of solids. The built-in features allow you to also study how various design parameters affect the performance of the battery, such as thickness and geometry of the electrodes and separators, the geometry of the current collectors and feeders, and more.
Generic Batteries
The workhorse of the Battery Design Module is the detailed model of the battery unit cells with positive electrode, negative electrode, and separator. With the generic description of porous electrodes, you can define any number of competing reactions in an electrode and also couple this to an electrolyte of an arbitrary composition. The module allows you to describe the pore electrolyte and the electrolyte in the separator, for any composition, with the theory for concentrated, dilute (Nernst–Planck equations), and supporting electrolytes in combination with porous electrode theory.
Perform various electrochemical analyses for batteries with the COMSOL® software.
Heterogeneous and Homogenous Models
Model the detailed structure of the porous electrodes and the pore electrolyte for a representative unit cell of a battery.
Growth of Solid Electrolyte Interface (SEI)
Model aging in a negative graphite electrode of a lithium-ion battery.
Diffusion-Induced Stress
Compute intercalation stresses and strains caused by expansion and contraction.
Short-Circuiting
Investigate an internal short circuit of a battery.
Pseudo-Dimension
Model the intercalation of lithium in the electrode particles.
Double-Layer Capacitance
Model electrochemical capacitors and nanoelectrodes.
NiMH and NiCd Batteries
Model batteries with alkaline binary (1:1) electrolytes.
Flow Batteries
Simulate lead-acid and vanadium flow batteries during an applied charge–discharge load cycle.
Metal Plating
Specify electrode host capacities to avoid lithium metal plating during high-rate charging.
Porosity Effects
Model chemical reactions influenced by species transport in porous media.
Impedance Spectroscopy
Study the harmonic response of a battery using physics-based high-fidelity models.
Lumped Models with Parameter Estimation
Define a simplified battery model based on a small set of lumped parameters that fit results from high-fidelity models to experimental results.
The Battery Design Module offers a set of specialized tools to simulate the performance of batteries under different operating conditions.
Simplified Battery Modeling
For faster thermal analysis of 3D battery packs, validated lumped (simplified) models can be used for each battery in a pack. Once validated, the lumped models may give excellent accuracy within a particular range of operation. The Battery Design Module contains lumped models that are physics-based and solve the electrochemical equations in multiple space dimensions.
The Single Particle Battery interface models the charge distribution in a battery using one separate single-particle model each for the positive and negative electrodes of the battery. The Lumped Battery interface makes use of a small set of lumped parameters for adding contributions for the sum of all voltage losses in the battery, stemming from ohmic resistances and, optionally, charge transfer and diffusion processes. Additionally, you can define a battery model based on an arbitrary number of electrical circuit elements with the Battery Equivalent Circuit interface.
Porous Electrodes with an Arbitrary Number of Electrochemical Reactions
Battery systems and chemistries are often burdened by unwanted side-reactions at the electrodes, and you can investigate their impact on charge and discharge cycles, as well as for self-discharge.
Typical by-reactions that you are able to model include hydrogen evolution, oxygen evolution, the growth of a solid electrolyte interface, metal plating, metal corrosion, and graphite oxidation.
Fully Transient and Impedance Spectroscopy Studies
Battery systems are often closed systems that are difficult to study during operation. Transient methods such as potential step, current interrupt, and impedance spectroscopy can be used to characterize a battery during operation.
By performing transient studies, you can run parameter estimation at different time scales and frequencies to separate ohmic, kinetics, transport, and other losses that may be responsible for battery aging. Using transient techniques, modeling, and parameter estimation, you can make very accurate estimations of the state of health of a battery system.
High-Fidelity Battery Modeling
The Lithium-Ion Battery interface is used to compute the potential and current distributions in a lithium-ion battery. Multiple intercalating electrode materials can be used, and voltage losses due to SEI layers are also included.
The Battery with Binary Electrolyte interface is used to compute the potential and current distributions in a generic battery. Multiple intercalating electrode materials can be used, and voltage losses due to film formation on the porous electrodes can also be included.
Intercalating Species and Transport in Pore Structures
The particles in porous battery electrodes can either be solid (Li-ion electrode) or porous (lead–acid, NiCd). In the case of solid particles, the porosity in the electrode is found between the packed particles. However, transport and reactions may occur in the solid particles for small atoms such as hydrogen and lithium atoms. These intercalating species are modeled with a separate diffusion-reaction equation defined along the radius of the solid particles. The flux of the intercalating species is coupled at the surface of the particles with the species that are transported in the pore electrolyte between the particles. The intercalation species and reactions are predefined for Li-ion batteries, but you can use the same functionality to model intercalation of hydrogen in, for example, NiMH batteries.
In the case of porous particles, a bimodal pore structure is obtained: a macroporous structure between the packed particles and a microporous structure inside the particles. The reaction–diffusion equations in the porous particles are defined in a similar fashion as for the intercalation of species in solid particles.
Built-In Thermodynamics and Material Properties
The battery material database included in the module contains entries for a number of common electrodes and electrolytes, substantially reducing the amount of work needed for creating new battery models.
One of the more time-consuming and error-prone steps in the modeling of battery systems is to gather input data and to use it consistently. For example, it is important that the positive and negative electrodes are defined in the same reference systems. The equilibrium electrode (half-cell) potentials have to be measured or calibrated to the same reference electrodes, electrolytes, and temperatures before they are incorporated in the same battery system model.
