

- Home
- Companies
- Edinburgh Instruments - TECHCOMP Group
- Applications
- Charge Carrier Recombination Dynamics ...
Charge Carrier Recombination Dynamics of Semiconductor Photocatalysts - Energy
In this application note the dynamics of charge carriers in copper-nitrogen-titanium oxide are studied using time-resolved photoluminescence spectroscopy on the FLS980 Photoluminescence Spectrometer.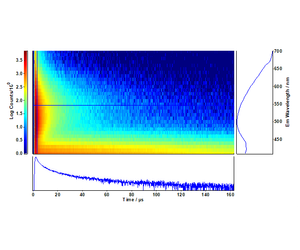
Photo-catalysis, the induction of chemical changes by absorption of light, is crucial for many environmental studies and sought for water splitting and hydrogen production,1,2 removal of pollutants,3,4 as well as artificial photosynthesis.5
With this wide spread of applications, earth-abundant photocatalysts are attracting extensive interest, especially based on anatase TiO2 due to its abundance,6–8 and low toxicity.5,9 Due to its wide bandgap at 3.2 eV, however, it is not a good absorber in the visible range. Viable routes to extend its absorption include doping with transition metals to induce defect states in the lattice and tune the bandgap towards the visible.
In this application note, by means of time-resolved photoluminescence spectroscopy, we study the dynamics of charge carriers in copper-nitrogen-titanium oxide (Cu-N-TiO2).
Time-resolved emission maps were performed in an FLS980 Fluorescence Spectrometer equipped with double excitation and emission monochromators, a photomultiplier tube detector (PMT-900) and a 450 W Xe lamp for steadystate spectral measurements. A Q-switched Nd: YAG laser (Continuum, Minilite) directly coupled to the spectrometer in L geometry was used as excitation in time-resolved measurements. The fundamental frequency of the laser was tripled, generating 355 nm light pulses of 4 ns pulse width at a repetition rate of 10 Hz and irradiance of 18 mW/cm2. Gratings blazed at 400 nm were used in the excitation and emission arms, with higher diffraction orders being filtered by the integrated long wave-pass filters in the FLS980.
Thin-films of pure and Cu-N-doped TiO2 samples were deposited on <100> silicon substrates by radio-frequency (RF) magnetron sputtering. High purity TiO2 and copper plates were used in a vacuumed deposition chamber under argon atmosphere. For PL measurements, the samples were placed on a front face sample holder utilising 45° orientation, while a 395 nm long-pass filter placed on the sample holder was used to eliminate scattering excitation light.
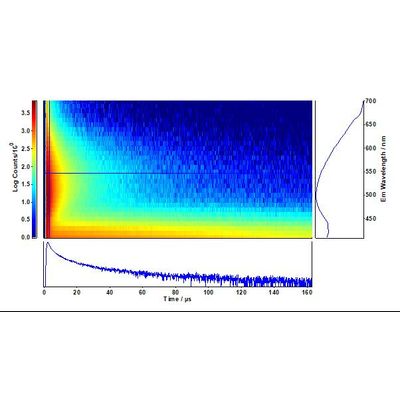
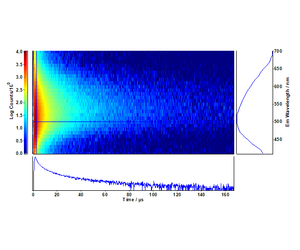
The distinctive decay kinetics of TiO2 and Cu-N-TiO2 are evident in the time-resolved emission maps of Figures 1 and 2, respectively. Particularly exciton emission at 410 nm in pure TiO2 is completely quenched in favour of the Cu-N defect states associated to the nano-columns formed at the surface of the deposited films.10,11
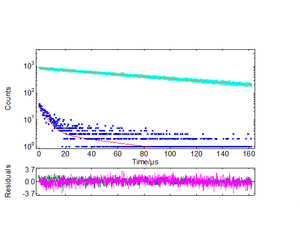
The fit of the decays at 410 nm and 550 nm were directly extracted from the maps of Figures 1 and 2. The long-lived exciton lifetime of pure TiO2 corresponds to a single
exponent of 93 μs, displayed in Figure 3. Although the long-lived emission fits well in an equation of the form, the recombination kinetics at 550 nm were better fit to stretched exponentials of the form.11
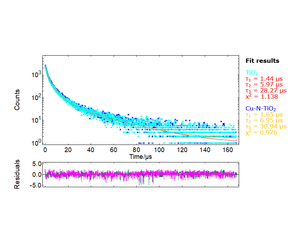
Reported TiO2 lifetimes vary from ns to ms depending on the preparation technique. Preparation techniques vary widely,12 with chemical vapour deposition (CVD) and RF magnetron sputtering being the most commonly used. While TiO2 powders of 60 nm to 3 μm crystal size reported 0.04 ns - 1.3 ns and up to 2.4 ns lifetimes in proportion to the crystal size,13-14 anatase films grown by CVD exhibited lifetimes in the range of 10 μs - 30 μs at room temperature and 77 K.15,16 Similar lifetimes in the range of 10 μs - 80 μs have been reported for nanostructured semiconductors such as oxidised porous silicon.10
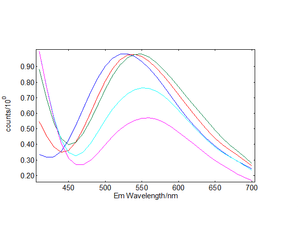
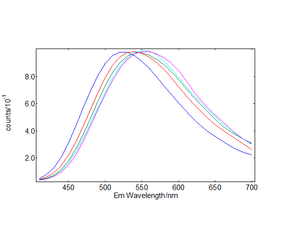
The time-resolved emission spectra (TRES) were readily obtained by the time-resolved emission maps in the range 32 μs - 40 μs in five bins of 2 μs width and are plotted in Figures 5 and 6. It can be clearly seen that the emission of the selftrapped exciton inherent in the pure TiO2 sample is completely quenched in the Cu-N-TiO2 sample.
In addition to the temporal evolution of the emission spectra that can be investigated, spectra free from artefacts can also be obtained with this technique. Care needs to be taken in the acquisition of excitation and emission spectra of TiO2 samples, weakly emitting under excitation with standard Xe lamps. As is the case of highly scattering samples such as PTFE, experimental artefacts can be superimposed with the emission of the sample.17-19 This can be circumvented by the use of appropriately powered lasers as excitation sources, as demonstrated in this application note.
Charge carrier recombination in thin-films of Cu-N-TiO2 was presented in this application note. The photoluminescence of the films was characterised by means of time-resolved spectroscopy and revealed the dynamics of charge carriers in TiO2 by addition of Cu and N. The use of high power pulsed laser sources in conjunction with FLS980 double monochromator fluorescence spectrometers enabled this study, while minimising the manifestation of artefacts commonly evidenced for weakly emitting samples.
- Ni, M., Leung, M. K. H., Leung, D. Y. C. & Sumathy, K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew. Sustain. Energy Rev. 11, 401–425 (2007).
- Moniz, S. J. A., Shevlin, S. A., Martin, D. J., Guo, Z.-X. & Tang, J. Visible-light driven heterojunction photocatalysts for water splitting – a critical review. Energy Environ. Sci. 8, 731–759 (2015).
- Wilhelm, P. & Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem. Photobiol. Chem. 185, 19–25 (2007).
- Ryu, J. & Choi, W. Substrate-Specific Photocatalytic Activities of TiO2 and Multiactivity Test for Water Treatment Application. Environ. Sci. Technol. 42, 294–300 (2008).
- Carp, O., Huisman, C. L. & Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 32, 33–177 (2004).
- Hernández-Alonso, M. D., Fresno, F., Suárez, S. & Coronado, J. M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2, 1231–1257 (2009).
- Haxel, G. B., Hedrick, J. B., Orris, G. J., Stauffer, P. H. & Hendley II, J. W. Rare earth elements: critical resources for high technology. (2002)
- Jewell Sally & Kimball Suzette M. USGS Minerals Information: Mineral Commodity Summaries. (U.S. Department of the Interior, U.S. Geological Survey, 2016).
- Schulz, J. et al. Distribution of sunscreens on skin. Adv. Drug Deliv. Rev. 54, Supplement, S157–S163 (2002).
- Vial, J. C. et al. Mechanisms of visible-light emission from electro-oxidized porous silicon. Phys. Rev. B 45, 14171–14176 (1992).
- El Koura, Z. et al. Synthesis and Characterization of Cu and N Codoped RF-Sputtered TiO2 Films: Photoluminescence Dynamics of Charge Carriers Relevant for Water Splitting. J. Phys. Chem. C 120, 12042–12050 (2016).
- Fujishima, A., Zhang, X. & Tryk, D. A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008).
- Fujihara, K., Izumi, S., Ohno, T. & Matsumura, M. Time-resolved photoluminescence of particulate TiO2 photocatalysts suspended in aqueous solutions. J. Photochem. Photobiol. Chem. 132, 99–104 (2000).
- Yamada, Y. & Kanemitsu, Y. Determination of electron and hole lifetimes of rutile and anatase TiO2 single crystals. Appl. Phys. Lett. 101, 133907 (2012).
- Sekiya, T., Tasaki, M., Wakabayashi, K. & Kurita, S. Relaxation process in anatase TiO2 single crystals with different colors. J. Lumin. 108, 69–73 (2004).
- Wakabayashi, K., Yamaguchi, Y., Sekiya, T. & Kurita, S. Time-resolved luminescence spectra in colorless anatase TiO2 single crystal. J. Lumin. 112, 50–53 (2005).
- Edinburgh Instruments. Comparison of Stray Light Performance for FLS980 Spectrometers with either Single or Double Monochromators. (Edinburgh Instruments).
- Jiang, X. et al. Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study. J. Phys. Chem. C 116, 22619–22624 (2012).
- Rex, R. E., Knorr, F. J. & McHale, J. L. Comment on ‘Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study’. J. Phys. Chem. C 117, 7949–7951 (2013)
