

h2e Power Systems Pvt. Ltd.
- Home
- Companies
- h2e Power Systems Pvt. Ltd.
- Products
- Solid Oxide Fuel Cell
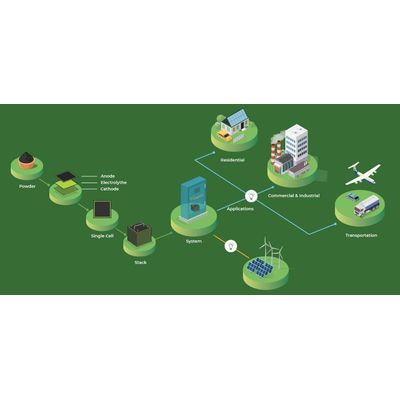
Solid Oxide Fuel Cell
A solid oxide fuel cell (SOFC) is a highly efficient electrochemical device that converts hydrogen and carbon monoxide from hydrocarbon fuels directly into electricity. Solid Oxide Fuel Cells use dense solid oxide as electrolyte material that conduct negative oxygen ions from cathode to anode. At anode, electrochemical oxidation of hydrogen (H2) or carbon monoxide (CO) takes place and at cathode oxygen reduction reaction takes place
Most popular related searches
solid-oxide fuel cell
solid oxide fuel cell
solid oxide fuel cell (SOFC)
fuel cell
catalyst precious metals
fuel cell SOFC
stationary power generator
heat recovery system
aviation fuel
power generation
- Operate at a higher temperature, do not require precious metal catalyst
- Operate on wide variety of hydrocarbon fuels
- Produce the highest electrical efficiency
- Most suited for continuous operation
- Can use heat recovery technologies for a total system efficiency of up to 85 percent
- Near zero emissions and quiet operation, with low maintenance
- Compared to existing generation technologies
They have a wide variety of applications form use as auxiliary in vehicles to stationary power generation. The higher operating temperature makes them a suitable candidate for application with cogeneration or combined hear and power which further increases the overall efficiency. Light hydrocarbon fuels such as methane, propane & butane can be internally reformed within the anode and heavier hydrocarbon fuels such as gasoline, diesel, jet fuel or biofuels can be externally reformed.
